The structure of a one-electron oxidized Mn(iii)-bis(phenolate)dipyrrin radical complex and oxidation catalysis control via ligand-centered redox activity†
Abstract
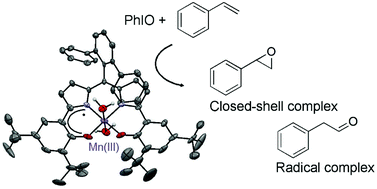
The tetradentate ligand dppH3, which features a half-porphyrin and two electron-rich phenol moieties, was prepared and chelated to manganese. The mononuclear Mn(III)-dipyrrophenolate complex 1 was structurally characterized. The metal ion lies in a square pyramidal environment, the apical position being occupied by a methanol molecule. Complex 1 displays two reversible oxidation waves at 0.00 V and 0.47 V vs. Fc+/Fc, which are assigned to ligand-centered processes. The one-electron oxidized species 1+ SbF6− was crystallized, showing an octahedral Mn(III) center with two water molecules coordinated at both apical positions. The bond distance analysis and DFT calculations disclose that the radical is delocalized over the whole aromatic framework. Complex 1+ SbF6− exhibits an Stot = 3/2 spin state due to the antiferromagnetic coupling between Mn(III) and the ligand radical. The zero field splitting parameters are D = 1.6 cm−1, E/D = 0.18(1), g⊥ = 1.99 and g∥ = 1.98. The dication 12+ is an integer spin system, which is assigned to a doubly oxidized ligand coordinated to a Mn(III) metal center. Both 1 and 1+ SbF6− catalyze styrene oxidation in the presence of PhIO, but the nature of the main reaction product is different. Styrene oxide is the main reaction product when using 1, but phenylacetaldehyde is formed predominantly when using 1+ SbF6−. We examined the ability of complex 1+ SbF6− to catalyze the isomerization of styrene oxide and found that it is an efficient catalyst for the anti-Markovnikov opening of styrene oxide. The formation of phenylacetaldehyde from styrene therefore proceeds in a tandem E–I (epoxidation–isomerization) mechanism in the case of 1+ SbF6−. This is the first evidence of control of the reactivity for styrene oxidation by changing the oxidation state of a catalyst based on a redox-active ligand.

 Please wait while we load your content...
Please wait while we load your content...