Carbon-based two electron σ-donor ligands beyond classical N-heterocyclic carbenes
Abstract
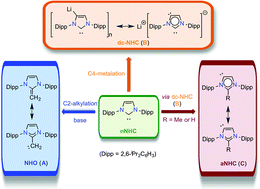
N-Heterocyclic carbenes (NHCs) are an important class of compounds that are indeed regarded as most versatile carbon-donor ligands in transition metal and organometallic catalysis. In addition, NHCs are also capable of stabilizing a variety of highly reactive main group compounds with intriguing properties. The enormous success of NHCs prompted the investigation of other carbon-based neutral ligands with additional structural and electronic features. Reactivity studies of NHCs and their complexes unveiled routes to new carbon donor ligands. Such NHC-derived ligands can be grouped into three categories: (i) N-heterocyclic olefins (the donor site is extended by an intervening carbon atom), (ii) ditopic carbanionic-NHCs (an additional coordination site is generated by the deprotonation of an NHC), and (iii) abnormal-NHCs (the carbene center of an NHC is moved to different positions). This article summarizes the recent advances in NHC chemistry of compounds featuring the aforementioned ligands, which are exclusively derived by the functionalization of NHCs, 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (IPr) in particular.


 Please wait while we load your content...
Please wait while we load your content...