Antimony(v) cations for the selective catalytic transformation of aldehydes into symmetric ethers, α,β-unsaturated aldehydes, and 1,3,5-trioxanes†
Abstract
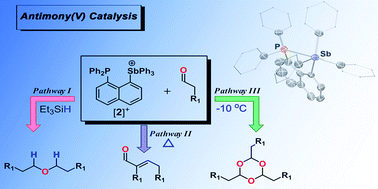
1-Diphenylphosphinonaphthyl-8-triphenylstibonium triflate ([2][OTf]) was prepared in excellent yield by treating 1-lithio-8-diphenylphosphinonaphthalene with dibromotriphenylstiborane followed by halide abstraction with AgOTf. This antimony(V) cation was found to be stable toward oxygen and water, and exhibited exceptional Lewis acidity. The Lewis acidity of [2][OTf] was exploited in the catalytic reductive coupling of a variety of aldehydes into symmetric ethers of type L in good to excellent yields under mild conditions using Et3SiH as the reductant. Additionally, [2][OTf] was found to selectively catalyze the Aldol condensation reaction to afford α-β unsaturated aldehydes (M) when aldehydes with 2 α-hydrogen atoms were used. Finally, [2][OTf] catalyzed the cyclotrimerization of aliphatic and aromatic aldehydes to afford the industrially-useful 1,3,5 trioxanes (N) in good yields, and with great selectivity. This phosphine–stibonium motif represents one of the first catalytic systems of its kind that is able to catalyze these reactions with aldehydes in a controlled, efficient manner. The mechanism of these processes has been explored both experimentally and theoretically. In all cases the Lewis acidic nature of the antimony(V) cation was found to promote these reactions.

 Please wait while we load your content...
Please wait while we load your content...