Characterization of a monocyanide model of FeFe hydrogenases – highlighting the importance of the bridgehead nitrogen for catalysis†
Abstract
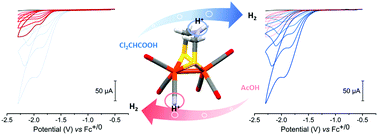
An azadithiolate bridged monocyanide derivative [Fe2(adt)(CO)5(CN)]− of [Fe2(adt)(CO)4(CN)2]2− has been prepared and extensively characterized as a model of the [FeFe]-hydrogenase active site, using a combination of FTIR spectroscopy, electrochemical methods and catalytic assays with chemical reductants. The presence of two basic nitrogen sites opens up multiple protonation pathways, enabling catalytic proton reduction. To our knowledge [Fe2(adt)(CO)5(CN)]− represents the first example of a cyanide containing [FeFe]-hydrogenase active site mimic capable of catalytic H2 formation in aqueous media.

 Please wait while we load your content...
Please wait while we load your content...