Antimony oxofluorides – a synthesis concept that yields phase pure samples and single crystals†
Abstract
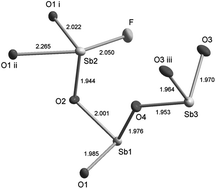
The single crystals of the new isostructural compounds Sb3O4F and Y0.5Sb2.5O4F and the two previously known compounds M-SbOF and α-Sb3O2F5 were successfully grown by a hydrothermal technique at 230 °C. The new compound Sb3O4F crystallizes in the monoclinic space group P21/c; a = 5.6107(5) Å, b = 4.6847(5) Å, c = 20.2256(18) Å, β = 94.145(8)°, z = 4. The replacing part of Sb with Y means a slight increase in the unit cell dimensions. The compounds M-SbOF and α-Sb3O2F5 have not been grown as single crystals before and it can be concluded that hydrothermal synthesis has proved to be a suitable technique for growing single crystals of antimony oxofluorides because of the relatively low solubility of such compounds compared to other antimony oxohalides that most often have been synthesised at high temperatures by solid state reactions or gas–solid reactions.

 Please wait while we load your content...
Please wait while we load your content...