A carboxylate-bridged Ni II8 cluster with a distorted cubane topology: structure, magnetism and density functional studies†
Abstract
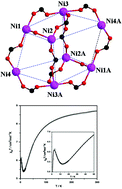
Using a dicarboxylate ligand appended with (2-pyridyl)ethylamine unit, a new cluster [NiII8(L4)6(DMF)2(CH3OH)2(H2O)6][ClO4]4·2CH3OH·2CH3CO2C2H5 (1) [L4(2−): 3-[N-{2-(pyridin-2-yl)ethyl}amino]-bis(propionate)] has been synthesized, through ‘coordination-driven self-assembly’. The crystal structure of 1 reveals a centrosymmetric octanuclear carboxylate-bridged nickel(II) tetracation, with a distorted cubane topology. The four crystallographically independent nickel(II) centres differ markedly in their coordination environment. Magnetic studies (2–300 K) reveal that in 1 the net magnetic-exchange is antiferromagnetic. Based on geometric parameters associated with two interacting nickel(II) centres, six magnetic-exchange coupling constants (J values) were considered for magnetic data analysis. Notably, 1 provides the first example of a NiII8 cluster (i) bridged solely by carboxylates in three bridging modes (monatomic, syn–anti and anti–anti), (ii) exhibiting four ferromagnetic and two antiferromagnetic magnetic-interactions and (iii) demonstrating a good agreement between six J values (obtained from experimental data analysis) and those obtained from DFT calculations, at the B3LYP-level of theory.

 Please wait while we load your content...
Please wait while we load your content...