Double-decker bis(tetradiazepinoporphyrazinato) rare earth complexes: crucial role of intramolecular hydrogen bonding†
Abstract
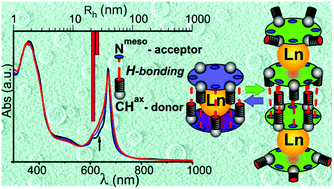
A series of homoleptic bis{tetrakis(5,7-bis(4-tert-butylphenyl)-6H-1,4-diazepino)[2,3-b,g,l,q]porphyrazinato}lanthanide sandwich complexes [tBuPhDzPz]2Ln (Ln = Lu, Er, Dy, Eu, Nd, Ce, La) were prepared and their physicochemical properties were studied to gain insight into the nature of specific interactions in diazepinoporphyrazines. The effect of annulated diazepine moieties and the Ln ionic radius on the properties of the complexes was investigated in comparison with double-decker phthalocyanines. A combination of experimental and theoretical studies revealed the presence of two types of hydrogen bonding interactions in the metal-free porphyrazine and the corresponding sandwich complexes, namely, interligand C–Hax⋯Nmeso hydrogen bonding and O–H⋯NDz ligand–water interaction. The interligand hydrogen bonding imparts high stability of the ligand dimer and the double-decker compounds in a reduced state. This work is the first comprehensive investigation into the fundamental understanding of the unusual properties of diazepine-containing macroheterocycles.


 Please wait while we load your content...
Please wait while we load your content...