Ni(ii)/Cu(ii)/Zn(ii) 5,5-diethylbarbiturate complexes with 1,10-phenanthroline and 2,2′-dipyridylamine: synthesis, structures, DNA/BSA binding, nuclease activity, molecular docking, cellular uptake, cytotoxicity and the mode of cell death†
Abstract
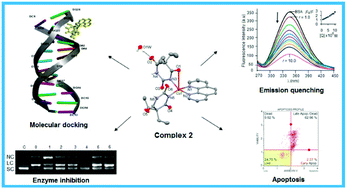
New 5,5-diethylbarbiturate (barb) complexes of Ni(II), Cu(II) and Zn(II) with 1,10-phenanthroline (phen) and 2,2′-dipyridylamine (dpya), namely [Ni(phen-κN,N′)3]Cl(barb)·7H2O (1), [Cu(barb-κN)(barb-κ2N,O)(phen-κN,N′)]·H2O (2), [Cu(barb-κN)2(phen-κN,N′)] (2a), [Zn(barb-κN)2(phen-κN,N′)]·H2O (3), [Ni(barb-κ2N,O)(dpya-κN,N′)2]Cl·2H2O (4), [Cu(barb-κ2N,O)2(dpya-κN,N′)]·2H2O (5) and [Zn(barb-κN)2(dpya-κN,N′)] (6), were synthesized and characterized by elemental analysis, UV-vis, FT-IR and ESI-MS. The structures of the complexes were determined by X-ray crystallography. Notably, 3 and 6 were fluorescent in MeOH : H2O at rt. The interaction of the complexes with fish sperm (FS) DNA and bovine serum albumin (BSA) was investigated in detail by various techniques. The complexes exhibited groove binding along with a partial intercalative interaction with DNA, while the hydrogen bonding and hydrophobic interactions played a major role in binding to BSA. It is noteworthy that 2 exhibited the highest affinity towards DNA and BSA. Enzyme inhibition assay showed that 1–4 show a preference for both A/T and G/C rich sequences in pUC19 DNA, while 5 and 6 display a binding specificity to the G/C and A/T rich regions, respectively. These findings were further supported by molecular docking. The cellular uptake studies suggested that 2 was deposited mostly in the membrane fraction of the cells. Among the present complexes, 2 exhibited a very strong cytotoxic effect on A549, MCF-7, HT-29 and DU-145 cancer cells, being more potent than cisplatin. Moreover, 2 induces cell death through the apoptotic mode obtained by flow cytometry.

 Please wait while we load your content...
Please wait while we load your content...