Chirality and catalysis with aromatic N-fused heterobicyclic carbenes
Abstract
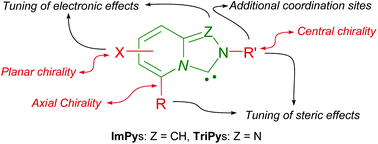
The benzoannulation of the most common families of aromatic NHCs, imidazol-2-ylidenes and 1,2,4-triazol-3-ylidenes, results in heterobicyclic imidazo[1,5-a]pyridin-3-ylidenes (ImPy's) and [1,2,4]triazolo[4,3-a]pyridin-3-ylidenes (TriPy's), characterized by a bridged N atom. These are versatile platforms that offer multiple possibilities for the modulation of the steric and electronic properties of the carbene ligand and/or organocatalyst, and offer also diverse opportunities for the introduction of several types of chiralities. In this paper the different families of chiral ImPy and TriPy carbenes and their application in asymmetric catalysis will be discussed.

 Please wait while we load your content...
Please wait while we load your content...