Thermodynamic study of the complexation between Nd3+ and functionalized diacetamide ligands in solution†
Abstract
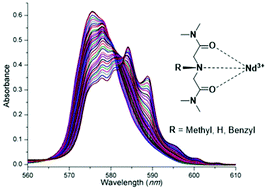
A series of amine functionalized ligands, including 2,2′-(benzylazanediyl)bis(N,N′-dimethylacetamide) (BnABDMA), 2,2′-azanediylbis(N,N′-dimethylacetamide) (ABDMA), and 2,2′-(methylazanediyl)bis(N,N′-dimethylacetamide) (MABDMA), are synthesized for the thermodynamic study of their complexation with Nd3+ ions. Their complexation in solution is investigated using potentiometry, spectrophotometry, calorimetry, and electrospray ionization mass spectrometry. The results suggest that these ligands act as tridentate ligands. Furthermore, direct comparison between ABDMA and an analogous ether-functionalized ligand, 2,2′-oxybis(N,N′-dimethylacetamide) (TMDGA), showed that the amine functionalized ligand forms thermodynamically stronger complexes with Nd3+ ions than the ether-functionalized ligand. In addition, the amine functionalized ligand can allow the fine-tuning of the binding strength with metal ions via substitution on the central amine N atom with different functional groups, which is not possible for ether functionalized ligands such as TMDGA.

 Please wait while we load your content...
Please wait while we load your content...