Transition metal mediated formation of dicationic diselenides stabilised by N-heterocyclic carbenes: designed synthesis†
Abstract
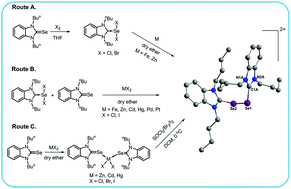
Reactions of the N-heterocyclic carbene derivatives of selenium(II) dihalides, LSeX2 (X = Cl, Br; L = 1,3-dibutylbenzimidazol-2-ylidene, C15H22N2) (4b, 4b′), L′SeX2 (X = Cl, Br; L′ = 1,3-dinpropylbenzimidazol-2-ylidene, C13H18N2) (4c, 4c′) and L′′SeX2 (X = Cl, Br; L′′ = 1,3-diipropylbenzimidazol-2-ylidene, C13H18N2) (4d, 4d′) with iron led to the formation of dicationic diselenides, [L2Se22+]2[{FeCl4}−]3Cl− (7), [L2Se22+][FeBr4]−Br− (8), [L′2Se22+][FeCl4]−Cl− (9), [L′2Se22+][FeBr4]−Br− (10), [L′′2Se22+][FeCl4]−Cl− (11) and [L′′2Se22+][FeBr4]−Br− (12). The reactions of LSeX2 with copper gave selone (LSe) complexes of cuprous halides, [(LSe)2CuBr] (13) and [(LSe)2(CuI)2] (14) respectively. However, LSeX2 (X = Cl, Br, I) (4b–4b′′) on treatment with zinc gave both the dicationic diselenides, [L2Se22+]Y [Y = {ZnCl3(H2O)2}2− (15), {ZnBr4}2− (17)] and complexes, [(LSe)2ZnX2] (16, 18, 20). The plausible mechanism for the formation of dicationic diselenides, [L2Se22+], has been proposed and is supported by performing various test reactions. To validate these mechanisms, DFT calculations have been carried out. Interestingly, the reactions of the mixture of LSeX2/L′SeX2/L′′SeX2 and L′Se0/L′Se0/L′′Se0 with metal halides (FeCl3, ZnX2; X = Cl, Br, I; CdI2, HgI2, PdCl2, and PtCl2) produced dicationic diselenides, 7–12, 15, 17 and [L2Se22+]Y [Y = {ZnI4}2− (19), {CdI4}2− (22), {HgI4}2− (25), {PdCl4}2− (26), {PtCl4}2− (27)]. The methodology is more general than the previous method and gives better yields of [L2Se22+]Y. The controlled oxidation of complexes, [(LSe)2MX2] (M = Zn, Cd, Hg; X = Cl, Br, I) (16, 18, 20, 28–32) with halogenating agents (SOCl2/Br2/I2) also gave dicationic diselenides, 15, 17, 19, 22, 25 and [L2Se22+]Y [Y = {CdCl4}2− (21), {Hg2Cl6}2− (23), {HgBr4}2− (24)], proving to be the most general method for the synthesis of [L2Se22+]. Attempted syntheses of unsymmetrical dicationic diselenides, [LSe-SeL′]2+[FeCl4]−Cl− and dicationic ditelluride, [L2Te22+]2[{FeCl4}−]3Cl− by the reaction of LSeX2/LTeX2 (X = Cl, Br, I) with Fe metal were unsuccessful. This observation has been corroborated with DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...