‘Click’ generated 1,2,3-triazole based organosulfur/selenium ligands and their Pd(ii) and Ru(ii) complexes: their synthesis, structure and catalytic applications†
Abstract
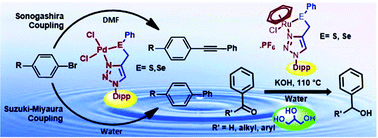
1-(2,6-Diisopropylphenyl)-4-(phenylthio/selenomethyl)-1H-1,2,3-triazole (L1/L2) was synthesized by a ‘Click’ reaction and treated with [Pd(CH3CN)2Cl2] for 5 h or [(η6-C6H6)RuCl(μ-Cl)]2 for 8 h (followed by reaction with NH4PF6) at room temperature, resulting in complexes [Pd(L)Cl2] (1 and 2) or [(η6-C6H6)Ru(L)Cl]PF6 (3 and 4) (L = L1 or L2), respectively. The four complexes (1–4) and ligands (L1 and L2) were characterized with 1H, 13C{1H} and 77Se{1H} NMR spectroscopy and high resolution mass spectrometry. The single crystal structures of 1–4 were solved. The geometry of Pd in 1 and 2 is distorted square planar. The Pd–S and Pd–Se bond distances in 1 and 2 are 2.277(3) and 2.384(6) Å respectively. In 3 and 4, there is a pseudo-octahedral “piano-stool” type disposition of donor atoms around Ru. The Ru–S and Ru–Se bond lengths in 3 and 4 are 2.3728(12) and 2.4741(6) Å respectively. The catalytic activity of complexes 1 and 2 was explored for Suzuki–Miyaura coupling (SMC) in water and the Sonogashira coupling reaction. For various aryl bromides, including deactivated ones, complexes 1 and 2 were found to be efficient catalysts for both couplings. The optimum loading of 1 and 2 required to catalyze both coupling reactions is of the order of 0.001–2 mol% of Pd. For SMC, no additive or phase transfer catalyst was added. For catalysis of the transfer hydrogenation (TH) of aldehydes and ketones, the half-sandwich Ru(II) complexes 3 and 4 were explored. Their optimum catalytic loading was found to be 0.1–0.4 mol% of Ru. For TH, both the water solvent and the glycerol hydrogen source are environmentally friendly. The catalytic efficiencies of 3 and 4 are comparable with those reported for other catalysts for TH carried out with 2-propanol or glycerol as a H-source. 1, with a sulfur ligand, is more efficient than 2 (Se analog) for both SMC and the Sonogashira coupling. The activities of 3 and 4 for TH are in the order Se > S.

 Please wait while we load your content...
Please wait while we load your content...