A 1,2,3-dithiazolyl-o-naphthoquinone: a neutral radical with isolable cation and anion oxidation states†
Abstract
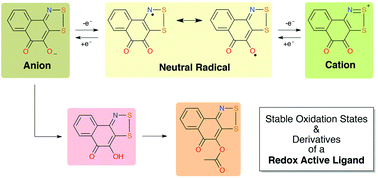
Under aprotic conditions, the reaction of 4-amino-1,2-naphthoquinone with excess S2Cl2 generates 4,5-dioxo-naphtho[1,2-d][1,2,3]dithiazol-2-ium chloride in a typical Herz condensation. By contrast, prior literature reports an imine (NH) product, 4,5-dioxo-1H-naphtho[1,2-d][1,2,3]dithiazole, for the same reaction performed in acetic acid. Herein, the cation product is isolated with four different counter-anions (Cl−, GaCl4−, FeCl4− and OTf−). Reduction of the cation generates a neutral radical 1,2,3-dithiazolyl-o-naphthoquinone, with potential ligand properties. Further reduction generates a closed shell anion, isolated as a water-stable Li+ complex and exhibiting O,O-bidentate chelation. The hydroxy (OH) isomer of the original imine (NH) product is reported, and this can be readily deprotonated and acylated (OAc). All species are structurally characterized. Solution redox behaviour and EPR are discussed where appropriate.

 Please wait while we load your content...
Please wait while we load your content...