Phosphine-ligated dinitrosyl iron complexes for redox-controlled NO release†
Abstract
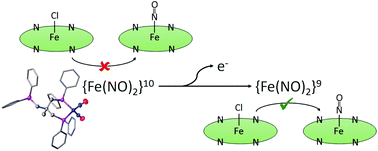
Here we present the syntheses and structural, spectroscopic, as well as electrochemical properties of four dinitrosyl iron complexes (DNICs) based on silicon- and carbon-derived di- and tripodal phosphines. Whereas CH3C(CH2PPh2)3 and Ph2Si(CH2PPh2)2 coordinate iron in a η2 – binding mode, CH3Si(CH2PPh2)3 undergoes cleavage of one Si–C bond to afford [Fe(NO)2(P(CH3)Ph2)2] at elevated temperatures. The complexes were characterized by IR spectroelectrochemistry as well as UV-vis measurements. The oxidized {Fe(NO)2}9 compounds were obtained by oxidation with (NH4)2[Ce(NO3)6] and their properties evaluated with Mössbauer and IR spectroscopy. Stability experiments on the complexes suggest that they are capable of releasing their NO-ligands in the oxidized {Fe(NO)2}9 but not in the reduced {Fe(NO)2}10 form. A detailed DFT analysis is provided in order to understand the electronic configurations and the complexes’ ability to release NO.

 Please wait while we load your content...
Please wait while we load your content...