The interaction of Hg2+ and trivalent ions with two new fluorescein bio-inspired dual colorimetric/fluorimetric probes†
Abstract
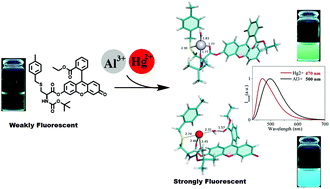
Two new luminescent compounds containing fluorescein–amino acid units have been designed and synthesized via an ester linkage between a fluorescein ethyl ester and Boc-Ser(TBDMS)-OH or Boc-Cys(4-MeBzl)-OH, and their photophysical properties have been explored. The optical response of both compounds (2 and 3) towards the metal ions Na+, K+, Hg+, Ag+, Ca2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+, Hg2+, Al3+, Fe3+, Ga3+and Cr3+ was investigated in pure acetonitrile and in acetonitrile/water mixtures. A strong CHEF (Chelation-Enhanced Fluorescence) effect was observed with all the trivalent metals and Hg2+ ions in both solvents. UV-vis absorption, steady state and time resolved emission spectroscopy methods were employed. The results show the formation of mononuclear complexes with Al3+, Fe3+, Ga3+, Cr3+, and Hg2+. Theoretical calculation using Density Functional Theory was performed in order to obtain atomistic insights into the coordination geometry of Al3+ and Hg2+ to the fluorescein 3, which is in accordance with the experimental stoichiometry results obtained in the Job's plot method. Among the active cations, the minimum detectable amount is under 1 μM for most of the cases in both absorption and fluorescence spectroscopy methods.


 Please wait while we load your content...
Please wait while we load your content...