Synthesis, structure and geometrically frustrated magnetism of the layered oxide-stannide compounds Fe(Fe3−xMnx)Si2Sn7O16†
Abstract
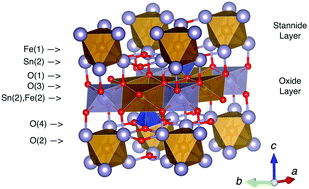
Fe4Si2Sn7O16 has a unique crystal structure that contains alternating layers of Fe2+ ions octahedrally coordinated by O (oxide layer) and Sn (stannide layer), bridged by SiO4 tetrahedra. The formula can be written as FeFe3Si2Sn7O16 to emphasise the distinction between the layers. Here, we report the changes in structure and properties as iron is selectively replaced by manganese in the oxide layer. Solid-state synthesis was used to produce polycrystalline samples of Fe(Fe3−xMnx)Si2Sn7O16 for x ≤ 2.55, the structures of which were characterised using high-resolution synchrotron X-ray and neutron powder diffraction. Single-crystal samples were also grown at x = 0.35, 0.95, 2.60 and characterised by single crystal X-ray diffraction. We show that manganese is doped exclusively into the oxide layer, and that this layer contains exclusively magnetically active high-spin M2+ transition metal cations; while the stannide layer only accommodates non-magnetic low-spin Fe2+. All samples show clear evidence of geometrically frustrated magnetism, which we associate with the fact that the topology of the high-spin M2+ ions in the oxide layer describes a perfect kagomé lattice. Despite this frustration, the x = 0 and x = 2.55 samples undergo long-range antiferromagnetic ordering transitions at 3.0 K and 2.5 K, respectively.

 Please wait while we load your content...
Please wait while we load your content...