Non-heme μ-Oxo- and bis(μ-carboxylato)-bridged diiron(iii) complexes of a 3N ligand as catalysts for alkane hydroxylation: stereoelectronic factors of carboxylate bridges determine the catalytic efficiency†
Abstract
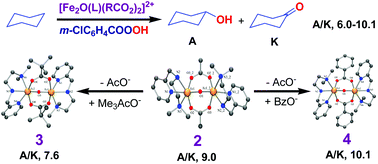
A series of non-heme (μ-oxo)bis(μ-dicarboxylato)-bridged diiron(III) complexes, [Fe2(O)(OOCH)2(L)2]2+1, [Fe2(O)(OAc)2(L)2]2+2, [Fe2(O)(Me3AcO)2(L)2]2+3, [Fe2(O)(OBz)2(L)2]2+4, [Fe2(O)(Ph2AcO)2(L)2]2+5 and [Fe2(O)(Ph3AcO)3(L)2]2+6, where L = N,N-dimethyl-N′-(pyrid-2-ylmethyl)ethylenediamine, OAc− = acetate, Me3AcO− = trimethylacetate, OBz− = benzoate, Ph2AcO− = diphenylacetate and Ph3AcO− = triphenylacetate, have been isolated and characterized using elemental analysis and spectral and electrochemical techniques. They have been studied as catalysts for the selective oxidation of alkanes using m-chloroperbenzoic acid (m-CPBA) as the oxidant. Complexes 2, 3, and 4 possess a distorted bioctahedral geometry in which each iron atom is coordinated to an oxygen atom of the μ-oxo bridge, two oxygen atoms of the μ-carboxylate bridge and three nitrogen atoms of the 3N ligand. In an acetonitrile/dichloromethane solvent mixture all the complexes display a d–d band characteristic of the triply bridged diiron(III) core, revealing that they retain their identity in solution. Upon replacing electron-donating substituents on the bridging carboxylates by electron-withdrawing ones the E1/2 value of the one-electron FeIIIFeIII → FeIIIFeII reduction becomes less negative. On adding one equivalent of Et3N to a mixture of one equivalent of the complex and an excess of m-CPBA in the acetonitrile/dichloromethane solvent mixture an intense absorption band (λmax, 680–720 nm) appears, which corresponds to the formation of a mixture of complex species. All the complexes act as efficient catalysts for the hydroxylation of cyclohexane with 380–500 total turnover numbers and good alcohol selectivity (A/K, 6.0–10.1). Adamantane is selectively oxidized to 1-adamantanol and 2-adamantanol (3°/2°, 12.9–17.1) along with a small amount of 2-adamantanone (total TON, 381–476), and interestingly, the sterically demanding trimethylacetate bridge around the diiron(III) centre leads to high 3°/2° bond selectivity; on the other hand, the sterically demanding triphenylacetate bridge gives a lower 3°/2° bond selectivity. A remarkable linear correlation between the pKa of the bridging carboxylate and TON for both cyclohexane and adamantane oxidation is observed, illustrating the highest catalytic activity for 3 with strongly electron-releasing trimethylacetate bridges.

 Please wait while we load your content...
Please wait while we load your content...