Ruthenium complexes of P-stereogenic phosphines with a heterocyclic substituent†
Abstract
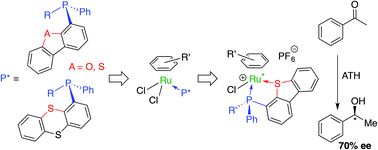
The synthesis via phosphine-boranes of 13 new optically pure P-stereogenic diarylphosphines P(Het)PhR (Het = 4-dibenzofuranyl (DBF), 4-dibenzothiophenyl (DBT), 4-dibenzothiophenyl-S,S-dioxide (DBTO2) and 1-thianthrenyl (TA); R = OMe, Me, i-Pr, Fc (ferrocenyl)) following the Jugé–Stephan method is described. The ligands were designed with the aim of having a heteroatom in a position capable of interacting with a metal upon coordination. The ligands and their precursors have been fully characterised, including the determination of two crystal structures of phosphine-boranes. Ru neutral complexes of the type [RuCl2(η6-arene)(κP-P)] (arene = p-cymene and methyl benzoate) have been prepared and characterised, including three crystal structure determinations. Treatment of solutions of the complexes with TlPF6 allowed the preparation of well-defined cationic complexes [RuCl(η6-arene)(κ2P,S-P)]PF6 for DBT- and TA-based phosphines. The complexes possess a stereogenic Ru atom and in most of the cases they are present as a single isomer in solution. All the Ru complexes have been used in the asymmetric transfer hydrogenation of acetophenone in refluxing 2-propanol, with good activities and up to 70% ee.

 Please wait while we load your content...
Please wait while we load your content...