Tuning the structure and solubility of nanojars by peripheral ligand substitution, leading to unprecedented liquid–liquid extraction of the carbonate ion from water into aliphatic solvents†
Abstract
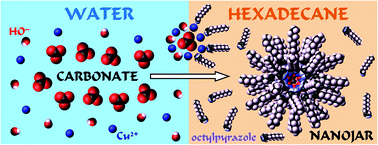
Nanojars, a novel class of neutral anion-incarcerating agents of the general formula [CuII(OH)(pz)]n (Cun; n = 27–31, pz = pyrazolate anion), efficiently sequester various oxoanions with large hydration energies from water. In this work, we explore whether substituents on the pyrazole ligand interfere with nanojar formation, and whether appropriate substituents could be employed to tune the solubility of nanojars in solvents of interest, such as long-chain aliphatic hydrocarbons (solvent of choice for large-scale liquid–liquid extraction processes) and water. To this end, we conducted a comprehensive study using 40 different pyrazole ligands, with one, two or three substituents in their 3-, 4- and 5-positions. The corresponding nanojars are characterized by single-crystal X-ray diffraction and/or electrospray-ionization mass spectrometry (ESI-MS). The results show that Cun-nanojars with various substituents in the pyrazole 4-position, including long chains, phenyl and CF3 groups, can be obtained. Straight chains are also tolerated at the pyrazole 3-position, and favor the Cu30-nanojar. Homoleptic nanojars, however, could not be obtained with phenyl or CF3 groups. Nevertheless, if used in mixture with the parent non-substituted pyrazole, sterically hindered pyrazoles do form heteroleptic nanojars. With 3,5-disubstituted pyrazoles, only heteroleptic nanojars are accessible. The crystal structure of novel nanojars (Bu4N)2[CO3⊂{Cu30(OH)30(3,5-Me2pz)y(pz)30−y}] (y = 14 and 15) is presented. We find that in contrast to the parent nanojar, which is insoluble in aliphatic solvents and water, nanojars with alkyl substituents are soluble in saturated hydrocarbon solvents, whereas nanojars based on novel pyrazoles, functionalized with oligoether chains, are readily soluble in water. Liquid–liquid extraction of carbonate from water under basic pH is presented for the first time.

 Please wait while we load your content...
Please wait while we load your content...