OFF–ON–OFF fluorescent response of N,N,N′,N′-tetrakis(1-isoquinolylmethyl)-2-hydroxy-1,3-propanediamine (1-isoHTQHPN) toward Zn2+†
Abstract
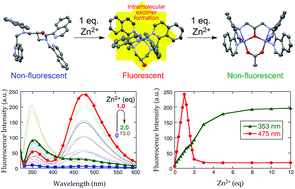
An isoquinoline-based ligand, N,N,N′,N′-tetrakis(1-isoquinolylmethyl)-2-hydroxy-1,3-propanediamine (1-isoHTQHPN), exhibits a fluorescence increase at 475 nm upon addition of 1 equiv. of Zn2+ (IZn/I0 = 12, ϕZn = 0.023). This fluorescence enhancement turns and then decreases sharply after the addition of more than 1 equiv. of Zn2+ reaching a constant minimum intensity with more than 2 equiv. of Zn2+. In contrast, the fluorescence intensity at 353 nm continues to increase until the signal saturates at ca. 5 equiv. of Zn2+. This observation can be explained by the formation of a fluorescent mononuclear complex ([Zn(1-isoHTQHPN)]2+) followed by a non-fluorescent dinuclear complex ([Zn2(1-isoTQHPN)]3+) at 475 nm during the titration of 1-isoHTQHPN with Zn2+. Both the mono- and dinuclear complexes were characterized by UV-vis, fluorescence, 1H NMR, ESI-MS, X-ray crystallography and TDDFT calculations. The fluorescence enhancement at 475 nm is Zn2+-specific; Cd2+ induces a much smaller emission increase (ICd/I0 = 3.7, ICd/IZn = 31%). The Zn2+/Cd2+ selectivity of the fluorescent response correlates with the difference in excimer-forming ability derived from the Cd–Nisoquinoline and Zn–Nisoquinoline bond distances.

 Please wait while we load your content...
Please wait while we load your content...