Electronic and steric Tolman parameters for proazaphosphatranes, the superbase core of the tri(pyridylmethyl)azaphosphatrane (TPAP) ligand†
Abstract
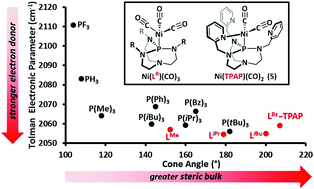
The Tolman electronic parameters (TEP) and cone angles were experimentally measured for a series of substituted proazaphosphatrane ligands by synthesizing their respective Ni(LR)(CO)3 complexes, where L = P(RNCH2CH2)3N and R = Me, iPr, iBu and Bz. The complexes Ni(LMe)(CO)3 (1), Ni(LiPr)(CO)3 (2), Ni(LiBu)(CO)3 (3) and Ni(LBz)(CO)3 (4) display CO vibrational frequencies (A1 mode) at 2057.0, 2054.6, 2054.9 and 2059.1 cm−1, respectively. The TEPs for the phosphine ligands in 1–3 are among the lowest measured, with values close to P(tBu)3 the most donating phosphine measured by Tolman. The cone angles of LR measured in 1–4 are 152, 179, 200 and 207° for R = Me, iPr, iBu and Bz, respectively. The substituted proazaphosphatranes have larger cone angles compared to the analogous trialkyl subsituted monophosphines. Our study demonstrates that while the cone angles have a significant dependence on R, all of the substituted proazaphosphatranes are strong electron donors. Additionally, in order to determine the electronic donor strength of our previously reported multidentate ligand, TPAP, Ni(TPAP)(CO)2 (5) (TPAP = tris(2-pyridylmethyl)azaphosphatrane) and Ni(LMe)2(CO)2 (6) were also synthesized and evaluated in a similar fashion.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...