Different structural preference of Ag(i) and Au(i) in neutral and cationic luminescent heteropolynuclear platinum(ii) complexes: Z (U)-shaped Pt2M2 type vs. trinuclear PtM2 type†
Abstract
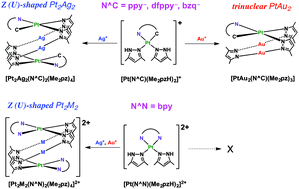
The reactions of monocationic Pt(II) complexes bearing N^C chelate ligands and Me2pzH, [Pt(N^C)(Me2pzH)2]PF6 (N^C = 2-phenylpyridinate (ppy−), 2-(2,4-difluorophenyl)pyridinate (dfppy−), benzo[h]quinolinate (bzq−); Me2pzH = 3,5-dimethylpyrazole), with Ag(I) ions gave Z (or U)-shaped neutral tetranuclear Pt2Ag2 complexes [Pt2Ag2(N^C)2(Me2pz)4], while those with Au(I) ions gave neutral trinuclear PtAu2 complexes [PtAu2(N^C)(Me2pz)3]. On the contrary, the reactions of the dicationic Pt(II) complex bearing a N^N chelate ligand and Me2pzH, [Pt(bpy)(Me2pzH)2](PF6)2 (bpy = 2,2′-bipyridine), with Ag(I) and Au(I) ions both gave Z (or U)-shaped dicationic tetranuclear Pt2M2 complexes, [Pt2M2(bpy)2(Me2pz)4](PF6)2 (M = Ag, Au). The structures of heteropolynuclear Pt(II) complexes were dominated by the nature of incorporated group 11 metal ions and the charge of complexes.

 Please wait while we load your content...
Please wait while we load your content...