Solvent influence on the thermodynamics for hydride transfer from bis(diphosphine) complexes of nickel†
Abstract
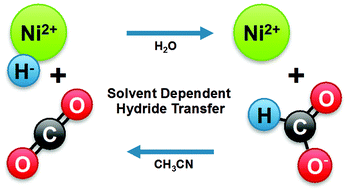
The thermodynamic hydricity of a metal hydride can vary considerably between solvents. This parameter can be used to determine the favourability of a hydride-transfer reaction, such as the reaction between a metal hydride and CO2 to produce formate. Because the hydricities of these species do not vary consistently between solvents, reactions that are thermodynamically unfavourable in one solvent can be favourable in others. The hydricity of a water-soluble, bis-phosphine nickel hydride complex was compared to the hydricity of formate in water and in acetonitrile. Formate is a better hydride donor than [HNi(dmpe)2]+ by 7 kcal mol−1 in acetonitrile, and no hydride transfer from [HNi(dmpe)2]+ to CO2 occurs in this solvent. The hydricity of [HNi(dmpe)2]+ is greatly improved in water relative to acetonitrile, in that reduction of CO2 to formate by [HNi(dmpe)2]+ was found to be thermodynamically downhill by 8 kcal mol−1. Catalysis for the hydrogenation of CO2 was pursued, but the regeneration of [HNi(dmpe)2] under catalytic conditions was unfavourable. However, the present results demonstrate that the solvent dependence of thermodynamic parameters such as hydricity and acidity can be exploited in order to produce systems with balanced or favourable overall thermodynamics. This approach should be advantageous for the design of future water-soluble catalysts.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...