Reduction of a diamidocarbene-supported borenium cation: isolation of a neutral boryl-substituted radical and a carbene-stabilized aminoborylene†
Abstract
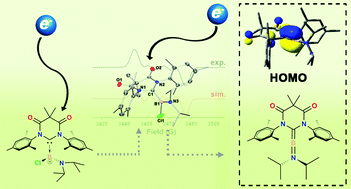
We have synthesized the first diamidocarbene (DAC)-supported borenium salt, [3][OTf], which was found to readily undergo two sequential 1-electron reductions. The first reduction forms a thermally robust, crystalline boryl-substituted DAC-centred radical (3˙) which has been fully characterized by XRD, EPR spectroscopy, and DFT analyses. The 1-electron reduction of 3˙ resulted in the formation of a DAC-supported aminoborylene, 4, which has been characterized computationally and by multinuclear NMR spectroscopy.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...