A series of new lanthanide fumarates displaying three types of 3-D frameworks†
Abstract
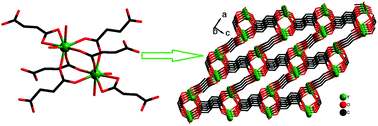
A series of lanthanide fumarates [Sm2(fum)3(H2fum)(H2O)2] (1, H2fum = fumaric acid), [Ln2(fum)3(H2O)4]·3H2O {Ln = Tb (2a), Dy (2b)} and [Ln2(fum)3(H2O)4] {Ln = Y (3a), Ho (3b), Er (3c), Tm (3d)} were prepared by the hydrothermal method and their structures were classified into three types. The 3-D framework of compound 1 contains a 1-D infinite [Sm–O–Sm]n chain built up from the connection of SmO8(H2O) polyhedra sharing edges via three –COO group bridges of fumarate ligands, which is further constructed into a 3-D network structure with three kinds of fumarate ligands. Compounds 2a–b are isostructural and consist of a 3-D porous framework with 0-D cavities for the accommodation of chair-like hexameric (H2O)6 clusters. Compounds 3a–d are isostructural and have a 3-D network structure remarkably different from those of 1 and 2a–b, due to the different coordination numbers for the Ln3+ ions and distinct fumarate ligand bridging patterns. A systematic investigation of seven lanthanide fumarates and five reported compounds revealed that the well-known lanthanide contraction has a significant influence on the formation of lanthanide fumarates. The magnetic properties of compounds 1, 2b and 3b–3d were also investigated.

 Please wait while we load your content...
Please wait while we load your content...