Impact of {Os(pap)2} in fine-tuning the binding modes and non-innocent potential of deprotonated 2,2′-bipyridine-3,3′-diol†
Abstract
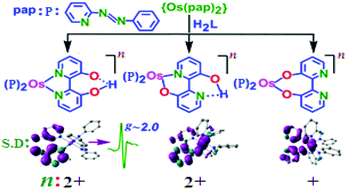
The reaction of ctc-OsII(pap)2Cl2 (pap = 2-phenylazopyridine, ctc = cis–trans–cis with respect to chlorides and pyridine/azo nitrogens of pap, respectively) and ambidentate 2,2′-bipyridine-3,3′-diol (H2L) leads to the simultaneous formation of isomeric [OsII(pap)2(HL−)]+ (2+/3+), seven-membered chelate containing OsII(pap)2(L2−) (4) and diastereomeric [{OsII(pap)2}2(μ-L2−)]2+ (5a2+ (meso, ΔΛ)/5b2+ (rac, ΔΔ/ΛΛ)). The reaction of 2,2′-biphenol (H2L′) and ctc-OsII(pap)2Cl2 yields OsII(pap)2(L′2−) (6), an analogue of 4. The identities of the newly designed complexes have been established by different analytical, spectroscopic and X-ray diffraction techniques. 1H-NMR spectra of the complexes and single crystal X-ray structures of selective derivatives [2]ClO4, [3]ClO4, [5a](ClO4)2, and 6 establish the retention of the tc-configuration of the precursor {Os(pap)2}. In isomeric 2+ and 3+, monodeprotonated HL− is linked to the {OsII(pap)2} fragment through N,N and N,O− donors, resulting in nearly planar five- and six-membered chelates with O–H⋯O− and O–H⋯N hydrogen bonds at its back face, respectively. The O−,O− donating L′2− extends a severely twisted seven-membered chelate with the {Os(pap)2} unit in 6. The N,O−/O−,N donors of deprotonated L2− bridge the two {OsII(pap)2} units in a symmetric fashion in 5a2+, forming two moderately twisted six-membered chelates. Though the deprotonation of the O–H⋯N hydrogen bond in 2+ by another unit of {OsII(pap)2} generates a diastereomeric mixture of 5a2+ and 5b2+, attempts to deprotonate the relatively stronger O–H⋯O− hydrogen bond in 2+ have failed. The isomeric 2+/3+, seven-membered chelate containing 4/6 and diastereomeric 5a2+/5b2+ exhibit distinctive 1H-NMR and absorption spectra as well as electrochemical responses. The pap (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) based two successive reductions and the participation of HL−, L2−, L′2− in the oxidation processes of the respective complexes have been revealed using EPR and DFT calculated MOs and Mulliken spin density plots at the intermediate paramagnetic states.
N) based two successive reductions and the participation of HL−, L2−, L′2− in the oxidation processes of the respective complexes have been revealed using EPR and DFT calculated MOs and Mulliken spin density plots at the intermediate paramagnetic states.

 Please wait while we load your content...
Please wait while we load your content...