Alteration of steric hindrance modulates glutathione resistance and cytotoxicity of three structurally related RuII-p-cymene complexes†
Abstract
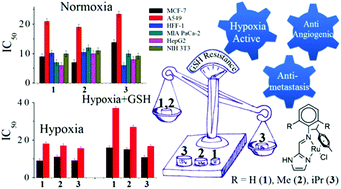
The effect of steric hindrance on reactivity towards biomolecules while designing RuII-η6-p-cymene based anticancer agents seems to be an important parameter in improving the activity and inducing resistance against glutathione (GSH) deactivation. Herein we present the structure, hydrolysis, anticancer activity and the effect of steric hindrance on deactivation by glutathione for three complexes, [RuII(η6-p-cym)(L1)(Cl)](PF6) (1), [RuII(η6-p-cym)(L2)(Cl)](PF6) (2) and [RuII(η6-p-cym)(L3)(Cl)](PF6) (3). The ligands L1–L3 are Schiff bases which show increasing substitution in a benzene ring, such that two ortho hydrogens are replaced by -methyl in 2 and by -isopropyl in 3. The cytotoxicity results strongly suggest that controlling the rate of hydrolysis through tuning of steric hindrance may be a feasible pathway to derive GSH resistant anticancer agents. The cellular studies show that all the three complexes show good blood compatibility (haemolysis <3%) and induce cellular death through caspase activation via the mitochondrial pathway. They have anti-angiogenic activity and prevent the healing of treated cells.

 Please wait while we load your content...
Please wait while we load your content...