Tuning of the sensing properties of luminescent Eu3+ complexes towards the nitrate anion†
Abstract
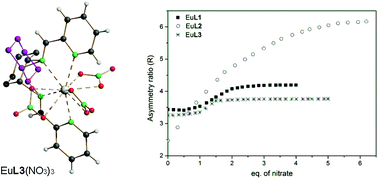
A new family of imine-based ligands containing pyridine or furan as an aromatic donating ring [N,N′-bis(2-pyridylmethylidene)-1,2-(R,R + S,S)-cyclohexanediamine, L1; N,N′-bis(2-furanylmethylidene)-1,2-(R,R + S,S)-cyclohexanediamine, L2 and N,N′-bis(2-thienylmethylidene)-1,2-(R,S)-cyclohexanediamine, L3] has been prepared in high yield by means of an easy synthetic protocol. Their trifluoromethansulphonate (CF3SO3−, OTf−) Eu(III) complexes have been employed for luminescence sensing of the NO3− anion in an anhydrous acetonitrile solution. Spectrophotometric titrations have been carried out to define the speciation in the solution and study the formation of ternary species occurring with the addition of NO3− anions. The sensing response towards this anion is strongly dependent on the nature of the ligand, the stoichiometry of the complexes and their concentration.

 Please wait while we load your content...
Please wait while we load your content...