Synthesis, structure and luminescent properties of lanthanide fluoroalkoxides†
Abstract
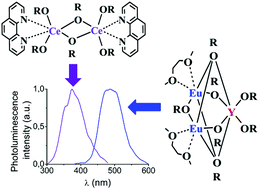
Alkoxides [Ln(OR)3(DME)]2 (R = CH(CF3)2, Ln = Sm (1), Yb (2)), [Ce(OR)3(Phen)]2 (3) (Phen = 1,10-phenanthroline), [Ce(OR′)3(DME)2]2 (R′ = C(CF3)3) (4), {Gd(OR′)3(DME)2} (5), {Ln2[O(CF3)2C–C(CF3)2O]3} (Ln = Ce (6), Gd (7)), {Ce2[O(CF3)2C–C(CF3)2O]3(Phen)2} (8), and {Ce[O(CF3)2C–C(CF3)2O][O(CF3)2–C(CF3)2OH](Phen)2} (9) were synthesized by the reactions of silylamides Ln[N(SiMe3)2]3 with respective fluorinated alcohols. The heterovalent trinuclear complex {Sm2(μ2-OR)3(μ3-OR)2Sm(OR)2(THF)2.5(Et2O)0.5} (10) was obtained by treatment of SmI2(THF)2 with ROK. The reaction of europium(II) and yttrium(III) silylamides with ROH afforded the heterobimetallic alkoxide {Eu2(μ2-OR)3(μ3-OR)2Y(OR)2(DME)2} (11) containing divalent europium. The molecular structures of 1, 2, 3, 9, 10 and 11 were determined by X-ray analysis. All the prepared cerium derivatives as well as the europium–yttrium isopropoxide upon UV excitation exhibited photoluminescence in the regions of 370–425 (for Ce3+) and 485 nm (for Eu2+) which was assigned to 4d→5f transitions.


 Please wait while we load your content...
Please wait while we load your content...