Anticancer activity of a cis-dichloridoplatinum(ii) complex of a chelating nitrogen mustard: insight into unusual guanine binding mode and low deactivation by glutathione†
Abstract
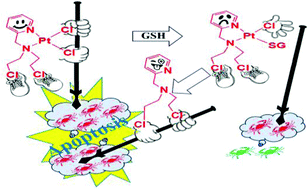
A pyridine ring containing a chelating nitrogen mustard ligand bis(2-chloroethyl)pyridylmethylamine hydrochloride (L2·HCl) was synthesized from bis(2-hydroxyethyl)pyridylmethylamine (L1) on reaction with thionyl chloride. Both the ligands upon reaction with cis-[PtCl2(DMSO)2] afforded square planar complexes cis-[PtCl2(L1)] (1) and cis-[PtCl2(L2)] (2) respectively. Both the complexes were characterized by NMR, IR, UV and elemental analysis. 2 crystallized in the P21/c space group. 2 shows greater solution stability than 1 in kinetic studies by 1H NMR. Both 1 and 2 bind the model nucleobase 9-ethylguanine (9-EtG) and form multiple mono-adducts. Existence of unusual N7,O6 chelated guanine bound 2 (2e) was traced. Binding studies of 2 with glutathione (GSH) show formation of a mono-adduct cis-[PtCl(L2)SG] (2c), which transformed within a day to give an aziridinium ion of L2 (2b) after loss of L2. In vitro cytotoxicity of ligands, complexes and the clinical anticancer drug cisplatin show that 2 is the most potent against MCF-7, A549 and MIA PaCa2 exhibiting IC50 values of 12.6 ± 0.8, 18.2 ± 1.8 and 4.2 ± 1.0 μM respectively. The in vitro cytotoxicity of 2 against MCF-7, A549 and MIA PaCa2 was also probed in hypoxia and in the presence and absence of added GSH. Even in the presence of excess GSH in hypoxia, 2 exhibits significant cytotoxicity against MIA PaCa2 and MCF-7 with IC50 of 4.4 ± 0.8 and 12.5 ± 1.1 μM respectively. Metal accumulation studies by ICP-MS display greater cellular internalization of 2, than 1 and cisplatin in MCF-7 cells. 2 arrests the cell cycle at sub G1 and G2/M phases in MCF-7 whereas cisplatin exhibits S phase arrest to be dominant with increase in concentration. Complex 2 exhibits a change in mitochondrial membrane potential, caspase activity and suggests apoptotic cell death through the intrinsic pathway. Moreover it is encouraging to find that 2 also restricts angiogenesis in chick embryo.

 Please wait while we load your content...
Please wait while we load your content...