Sulfurized metal borohydrides†
Abstract
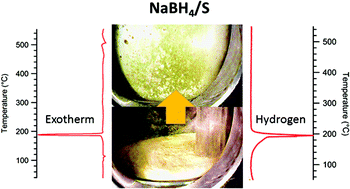
The reactions between metal borohydrides and elemental sulfur are investigated in situ during thermal treatment and are found to be highly exothermic (up to 700 J g−1). These reactions are exceptionally rapid, occurring below 200 °C, also resulting in the sudden release of substantial quantities of hydrogen gas. For NaBH4 this hydrogen release is pure, with no detectable levels of H2S or B2H6. The reaction results in the formation of an array of metal–boron–sulfur compounds. These MBH4–S compounds are interesting for possible uses in high energy applications (fuels or explosives), hydrogen generation, and metal–boron–sulfur precursors.

 Please wait while we load your content...
Please wait while we load your content...