Coinage metal coordination chemistry of stable primary, secondary and tertiary ferrocenylethyl-based phosphines†
Abstract
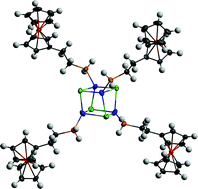
Ferrocene-based phosphines constitute an important auxiliary ligand in inorganic chemistry. Utilizing the (ferrocenylethyl)phosphines (FcCH2CH2)3−nHnP (Fc = ferrocenyl; n = 2, 1; n = 1, 2; n = 0, 3) the synthesis of a series of coordination complexes [(FcCH2CH2)3−nHnPCuCl]4 (n = 2, 1-CuCl; n = 0, 3-CuCl), [(FcCH2CH2)2HPCuCl] (2-CuCl), {[(FcCH2CH2)H2P]2AgCl}2 (1-AgCl), [(FcCH2CH2)2HPAgCl] (2-AgCl), [(FcCH2CH2)3PAgCl]4 (3-AgCl), [(FcCH2CH2)3PM(OAc)]4 (M = Cu, 3-CuOAc M = Ag, 3-AgOAc), [(FcCH2CH2)3−nHnPAuCl] (n = 1, 2-AuCl; n = 0, 3-AuCl), via the reaction between the free phosphine and MX (M = Cu, Ag and Au; X = Cl, OAc), is described. The reaction between the respective phosphine with a suspension of metal–chloride or -acetate in a 1 : 1 ratio in THF at ambient temperature affords coordinated phosphine-coinage metal complexes. Varying structural motifs are observed in the solid state, as determined via single crystal X-ray analysis of 1-CuCl, 3-CuCl, 1-AgCl, 3-AgCl, 3-CuOAc, 3-AgOAc, 2-AuCl and 3-AuCl. Complexes 1-CuCl and 3-CuCl are tetrameric Cu(I) cubane-like structures with a Cu4Cl4 core, whereas silver complexes with primary and tertiary phosphine reveal two different structural types. The structure of 1-AgCl, unlike the rest, displays the coordination of two phosphines to each silver atom and shows a quadrangle defined by two Ag and two Cl atoms. In contrast, 3-AgCl is distorted from a cubane structure via elongation of one of the Cl⋯Ag distances. 3-CuOAc and 3-AgOAc are isostructural with step-like cores, while complexes 2-AuCl and 3-AuCl reveal a linear geometry of a phosphine gold(I) chloride devoid of any aurophilic interactions. All of the complexes were characterized in solution by multinuclear 1H, 13C{1H} and 31P NMR spectroscopic techniques; the redox chemistry of the series of complexes was examined using cyclic voltammetry. This class of complexes has been found to exhibit one reversible Fe(II)/Fe(III) oxidation couple, suggesting the absence of electronic communication between the ferrocenyl units on individual phosphine ligands as well as between different phosphines on the polymetallic cores.

 Please wait while we load your content...
Please wait while we load your content...