μ3-Oxo stabilized by three metal cations is a sufficient nucleophile for enzymatic hydrolysis of phosphate monoesters†
Abstract
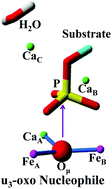
Diverse species have previously been proposed to be effective nucleophiles in the enzymatic hydrolysis of phosphate esters. A novel penta-metal cluster (two Fe3+ and three Ca2+) was recently discovered in the active site of PhoX alkaline phosphatase, with the revelation of the architecture of μ3-oxo bridging one Ca2+ and two antiferromagnetically coupled Fe3+. In this work, using density functional theory calculations, the μ3-oxo stabilized by three cations has been demonstrated to be a new type of effective nucleophile. The calculations give strong support to the “ping-pong” mechanism involving the nucleophilic attack of the μ3-oxo on the substrate phosphor and the subsequent hydrolysis of the covalent phospho–enzyme intermediate. A base mechanism with the μ3-oxo acting as a general base to activate an additional water molecule has further been demonstrated to be inaccessible. The results advance the understanding of the enzymatic hydrolysis of phosphate esters and may give important inspiration for the exploration of multinuclear biomimetic catalysts.

 Please wait while we load your content...
Please wait while we load your content...