N-heterocyclic phosphenium and phosphido nickel complexes supported by a pincer ligand framework†
Abstract
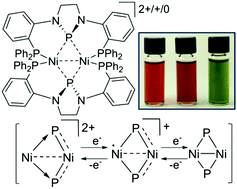
A chelating diphosphine ligand with a central N-heterocyclic phosphenium cation (NHP+) has been used to explore the coordination chemistry of NHPs with nickel. Treatment of the chlorophosphine precursor [PPP]Cl (1) with stoichiometric Ni(COD)2 affords (PPP)NiCl (8), which is best described as a NiII/NHP− phosphido complex formed via oxidative addition of the P–Cl bond. In contrast, treating [PPP]Cl (1) with excess Ni(COD)2 results in a mixture of the trimetallic complex (PPP)2Ni3Cl2 (9) and the reduced NHP-bridged dimer [(PPP)Ni]2 (10). Compound 9 is found to be a NiIINiIINi0 complex in which the two NHP ligands act as bridging NHP− phosphidos, while complex 10 is a NiINiI complex that is highly delocalized throughout the symmetric Ni2P2 core. In contrast, the reaction of [PPP][PF6] (11) with Ni(COD)2 affords an asymmetrically-bridged dication [(PPP)Ni]2[PF6]2 (12), which is found to contain two bridging NHP+ cations bridging two Ni0 centers. Comproportionation of 10 and 12 affords monocationic [(PPP)Ni]2[PF6] (13), completing the redox series. Nickel complexes 8–10 and 12 are largely similar to their Pd and Pt analogues, but a paramagnetic monocation such as 13 was not observed in the Pd and Pt case. Computational studies lend further insight into the electronic structure and bonding in complexes 8–10 and 12–13, and further support the potential redox non-innocent properties of NHP ligands.
- This article is part of the themed collections: Recipients of the Dalton Transactions UC Berkeley Lecture and Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...