Na+ diffusion kinetics in nanoporous metal-hexacyanoferrates†
Abstract
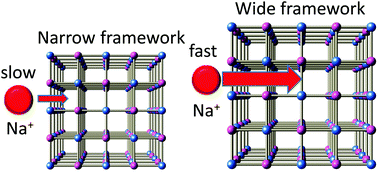
Metal-hexacyanoferrates (metal-HCFs) are promising candidates for cathode materials of sodium-ion secondary batteries (SIBs). Here, we systematically investigated Na+ diffusion constants (D) and the activation energies (Ea) of metal-HCFs against the framework size (= a/2). We found that the magnitude of D (Ea) systematically increases (decreases) with increases in a, indicating that steric hindrance plays a dominant role in Na+ diffusion.

 Please wait while we load your content...
Please wait while we load your content...