Analysis of the isomer ratios of polymethylated-DOTA complexes and the implications on protein structural studies†
Abstract
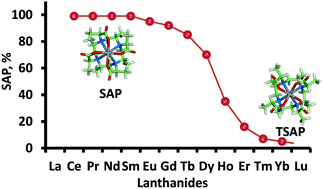
A rigidified and symmetrical polymethylated 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) ligand bearing four SSSS methyl groups in both the tetraaza ring and the acetate arms (SSSS-SSSS-M4DOTMA) was prepared. The isomer ratio of SSSS-SSSS-M4DOTMA complexed with a series of lanthanide ions was carefully investigated using RP-HPLC and NMR. A square antiprismatic (SAP) configuration was exclusively observed for the early lanthanides, while the twisted square antiprismatic (TSAP) geometry was preferred as the lanthanide ion size decreases. The late lanthanides preferentially adopted the TSAP geometry. One of the pendant arms was modified with a pyridyl disulfide group (SSSS-SSSS-M8SPy) for cysteine attachment and displayed a similar isomer trend as the parent compound, Ln-SSSS-SSSS-M4DOTMA. Covalent attachment to the ubiquitin S57C mutant showed resonances whose intensities are in agreement with the isomeric population observed by RP-HPLC. Furthermore, the NOE experiments combined with quantum chemical calculations have unequivocally demonstrated that the SAP of Pr-SSSS-SSSS-M4DOTMA and Pr-SSSS-SSSS-M8SPy, as well as the TSAP of Yb-SSSS-SSSS-M8SPy are more stable than their corresponding isomers.

 Please wait while we load your content...
Please wait while we load your content...