Systems biocatalysis: para-alkenylation of unprotected phenols†
Abstract
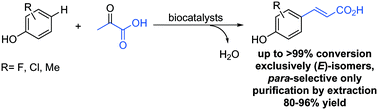
Commercially available phenol derivatives were transformed with pyruvate to form a new C–C bond leading to the corresponding para-coumaric acids and only one molecule of water as an innocent side product in buffer. The reaction was catalysed by a biocatalytic system consisting of two enzymatic steps, which were run simultaneously: (i) in the first step catalysed by a tyrosine phenol lyase the C–C coupling of phenol derivatives with pyruvate and ammonia yielded L-tyrosine derivatives, (ii) which were transformed in the second step to the final product via ammonia elimination catalysed by a tyrosine ammonia lyase. The reactions proceeded with exquisite regio- and stereoselectivity yielding just the para-products with the (E)-configuration. The method represents an efficient approach for the direct alkenylation of phenols on a preparative scale (up to 0.6 mmol) affording (E)-para-coumaric acids in excellent isolated yields without requiring chromatographic purification. Co-expression of the involved enzymes in a single host gave access to single catalyst preparation.


 Please wait while we load your content...
Please wait while we load your content...