Co3(OH)2(HPO4)2 as a novel photocatalyst for O2 evolution under visible-light irradiation†
Abstract
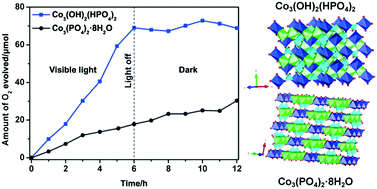
Cobalt phosphate has shown great potential as an electrocatalyst or a cocatalyst loaded on a photocatalyst for O2 evolution, but has not yet been successfully used as a photocatalyst for O2 evolution so far. Herein, a kind of cobalt phosphate, i.e., Co3(OH)2(HPO4)2 (Co3(PO4)2·2H2O), was prepared by a simple hydrothermal method, and was proved to be a novel, stable photocatalyst for O2 evolution under visible-light irradiation for the first time. The photocatalytic mechanism of Co3(OH)2(HPO4)2 was revealed by comprehensively comparing the physicochemical properties of Co3(OH)2(HPO4)2 with those of another kind of cobalt phosphate, i.e., Co3(PO4)2·8H2O, which was prepared by a precipitation method and showed little photocatalytic activity for O2 evolution under visible-light irradiation. The significantly improved photocatalytic activity of Co3(OH)2(HPO4)2, compared with that of Co3(PO4)2·8H2O, was mainly attributed to the synergistic promotion of the photocatalytic process by the following physicochemical properties of Co3(OH)2(HPO4)2, i.e., the distortion of Co2+ octahedra, the difference in electronic properties and the linkage of oxo bridges between the adjacent Co(1) and Co(2) octahedra. This work extended the application of cobalt phosphate in photocatalysis and presented an effective route to explore new O2-evolution photocatalysts by modifying the appropriate materials that were commonly employed as cocatalysts.

 Please wait while we load your content...
Please wait while we load your content...