Nitrate–nitrite equilibrium in the reaction of NO with a Cu-CHA catalyst for NH3-SCR†
Abstract
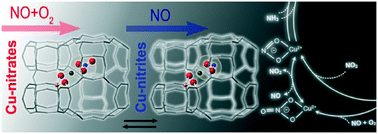
The equilibrium reaction between NO and Cu-nitrate, Cu(II)-NO3− + NO(g) ⇌ Cu(II)-NO2− + NO2(g), has been proposed to be a key step in the selective catalytic reduction of NO by ammonia (NH3-SCR) over Cu-CHA catalysts and points to the presence of Cu-nitrites. Whereas the formation of gaseous NO2 has been observed, a direct observation of Cu-nitrite groups under conditions relevant to NH3-SCR has been so far unsuccessful. In an effort to identify and characterize Cu-nitrites, the reaction between Cu-nitrates hosted in the CHA zeolite and NO is investigated by Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible spectroscopy (UV-vis) and X-ray absorption spectroscopy (XAS). We find that NO reacts with Cu-nitrates and that about half of the Cu-nitrate species are converted. After the reaction, the Cu(II) state is different from the original oxidized state. Analysis of XAS data indicates that the final state of the Cu-CHA catalyst is consistent with the partial conversion of the Cu-nitrate species to a bidentate Cu-nitrite configuration.


 Please wait while we load your content...
Please wait while we load your content...