Abstract
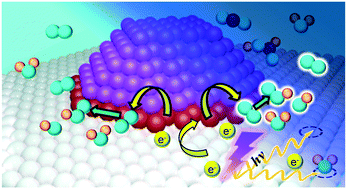
The potential for applying UV light pre-treatment to enhance the oxygen activation capacity of Au/TiO2 under ambient conditions was examined. Catalytic formic acid oxidation in an aqueous environment was employed as the test reaction. Pre-illuminating Au/TiO2 with UV light can amplify the catalytic formic acid oxidation rate by up to four times with the degree of enhancement governed by system parameters such as Au loading, pre-illumination time, and initial formic acid loading. X-ray photoelectron spectroscopy, photoluminescence spectroscopy and electrochemical assessment of the Au/TiO2 indicated light pre-illumination invokes photoexcited electron transfer from the TiO2 support to the Au deposits. The Au deposits then utilise the additional electrons to catalyse molecular oxygen activation and promote the oxidation reaction. Scanning tunneling spectroscopy analysis and first principle calculations indicated the Au deposits introduced new electronic states above the TiO2 valence band. The new electronic states were most intense at the Au–TiO2 interface suggesting the Au deposit:TiO2 perimeter may be the key region for oxygen activation. The current study has demonstrated that pre-illuminating Au/TiO2 with light can be used to augment reactions where oxygen activation is a critical component, such as for the oxidation of organic pollutants and for the oxygen reduction reaction in fuel cells or energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...