Selective CO2 methanation on Ru/TiO2 catalysts: unravelling the decisive role of the TiO2 support crystal structure†
Abstract
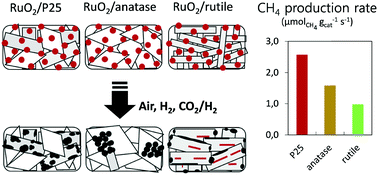
The catalytic hydrogenation of CO2 is a relevant strategy for mitigating CO2 emissions and its applicability relies on our ability to prepare catalysts that are highly active under mild conditions. Understanding and improving these tailored catalysts requires innovative materials synthesis routes and advanced methods of characterization. In this study, mono-dispersed 2 nm RuO2 nanoparticles were prepared as a stable colloidal suspension and deposited onto different titania supports by impregnation. Supported RuO2 nanoparticles are homogeneously dispersed at the surface of the titania supports. Then, upon annealing and reduction, metallic Ru nanoparticles are obtained, which are active in the hydrogenation of CO2 to CH4. However, depending on the crystal structure of the different TiO2 supports (anatase, rutile, and a mixture of both), the catalysts exhibited drastically diverse catalytic performances. An array of characterization tools (N2-physisorption, H2-chemisorption, HR-TEM, STEM-HAADF, 3D tomographic analysis, XRD, and XPS) was used to unravel the origin of this support effect. It appeared that catalytic behaviour was related to profound morphological changes occurring during the annealing step. In particular, advanced electron microscopy techniques allow visualisation of the consequences of RuO2 nanoparticle mobility onto titania. It is shown that RuO2 sinters heavily on anatase TiO2, but spreads and forms epitaxial layers onto rutile TiO2. On anatase, large Ru chunks are finally obtained. On rutile, the formation of a particular “rutile-TiO2/RuO2/rutile-TiO2 sandwich structure” is demonstrated. These phenomena – along with the relative thermal instability of the supports – explain why the catalysts based on the commercial P25 titania support outperform those based on pure crystalline titania. The study opens new perspectives for the design of highly active CO2 methanation catalysts.


 Please wait while we load your content...
Please wait while we load your content...