Insights into the mechanism for ring-opening polymerization of lactide catalyzed by Zn(C6F5)2/organic superbase Lewis pairs†
Abstract
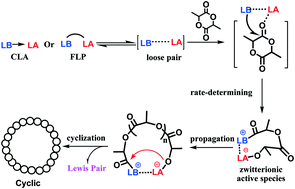
The mechanism for ring-opening polymerization (ROP) of lactide catalyzed by Zn(C6F5)2–organic superbase Lewis pairs was investigated in the present work. Common organic superbases 4-dimethylaminopyridine (DMAP), 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene (MesNHC), 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU), and 7-methyl-1,5,7-triazabicyclo[4.4.0]decane-5-ene (MTBD) were selected as the Lewis bases to work cooperatively with Zn(C6F5)2. It was demonstrated that less sterically bulky DMAP could coordinate with the zinc atom and form classical Lewis acid–base adducts (CLA), whereas frustrated Lewis pairs (FLP) were obtained when bulky MTBD and DBU were used. Investigation into the polymerization behavior of lactide showed that the frustrated Lewis pair Zn(C6F5)2/MTBD exhibited much higher activity in the polymerization of lactide and much lower temperature dependence compared with the Lewis adduct Zn(C6F5)2/DMAP. Furthermore, there is a direct relationship between polymerization activity and the degree of “frustration”. The initiation reaction was further explored by in situ NMR at variable temperature. The interactions between Zn(C6F5)2 and the organic base in both CLA and FLP would be impaired at high temperature, and lactide was activated electronically by coordination to the Lewis acid Zn(C6F5)2. In addition, NMR and MALDI-TOF analyses showed that the active species are zwitterionic species, in which each chain end bears one amine and one Zn(C6F5)2 moiety, respectively, and Zn(C6F5)2 associated with the amine closely. Based on these results, a possible mechanism involving bifunctional activation was proposed. Further kinetic studies showed that increasing the reaction temperature and solvent polarity can significantly enhance the chain initiation rate. These experimental results also demonstrated that the polymerization was initiated through the proposed mechanism.

 Please wait while we load your content...
Please wait while we load your content...