Demethylation of vanillic acid by recombinant LigM in a one-pot cofactor regeneration system†
Abstract
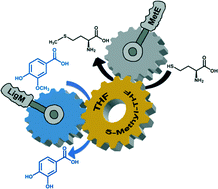
In recent years, by investigating bacterial degradation of lignin, interesting enzymatic activities aimed at breaking specific linkages were identified. In this work we focused on the tetrahydrofolate (THF)-dependent O-demethylase LigM from the bacterium Sphingobium sp. strain SYK-6 which converts vanillic acid to protocatechuic acid (PCA). The recombinant LigM was overexpressed in E. coli up to 80 mg L−1 fermentation broth, and the main kinetic parameters on the best substrate vanillic acid (kcat = 0.19 s−1 and Km = 54 μM) and the substrate specificity were identified. The substrate preference was rationalized using a three-dimensional model of LigM structure in complex with THF, which also allowed us to propose a reaction mechanism. LigM efficiently converts vanillic acid into PCA but the reaction requires a 10-fold molar excess of the THF cofactor. In order to limit the cofactor consumption, the plant methionine synthase MetE enzyme was also overexpressed in E. coli and used in combination with LigM. Under optimized conditions, the dual enzyme system produced 5 mM PCA using 0.1 mM THF only, a 500-fold decrease in the cofactor : substrate molar ratio compared to the mono-enzyme process. This represents the first regeneration method for THF in a biocatalytic process.

 Please wait while we load your content...
Please wait while we load your content...