H5PMo10V2O40 immobilized on functionalized chloromethylated polystyrene by electrostatic interactions: a highly efficient and recyclable heterogeneous catalyst for hydroxylation of benzene†
Abstract
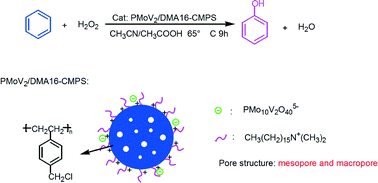
H5PMo10V2O40 (PMoV2) was immobilized on N,N-dimethylhexadecylamine functionalized chloromethylated polystyrene (DMA16-CMPS) by electrostatic interaction and used as a catalyst for the direct hydroxylation of benzene to phenol with H2O2. The results of FT-IR, XRD and TGA indicated that PMoV2 was immobilized and finely dispersed on DMA16-CMPS, and the structure was well preserved. UV-vis DRS, EPR and XPS confirmed the presence of V4+ in the catalyst due to electronic interactions between PMoV2 and DMA16-CMPS. The textural and morphological properties of the catalyst were characterized by N2 adsorption–desorption, SEM and TEM. The hydrophobicity of the catalyst was also characterized by contact angle measurement. PMoV2/DMA16-CMPS exhibited excellent catalytic performance with 22.1% conversion of benzene and a phenol yield of 21.9% at 65 °C. The high catalytic performance of PMoV2/DMA16-CMPS is attributed to high dispersion of PMoV2 on mesoporous and macroporous DMA16-CMPS, good benzene adsorption and phenol desorption ability, and V5+/V4+ redox pairs in the catalyst. Meanwhile, PMoV2/DMA16-CMPS has the advantages of facile recovery and recycling.

 Please wait while we load your content...
Please wait while we load your content...