The effect of phosphorus on the catalytic performance of nickel oxide in ethane oxidative dehydrogenation†
Abstract
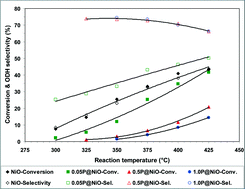
Surface-phosphated NiO catalysts with different phosphorus contents were prepared and used for ethane oxidative dehydrogenation (ODH) in the temperature range from 300 to 425 °C. The catalysts were characterized by nitrogen adsorption at −196 °C, XRD, ICP-OES, XPS, TEM, and Raman spectroscopy. They were also characterized by in situ electrical conductivity measurements at various temperatures and oxygen partial pressures, and the temporal response of the electrical conductivity to sequential exposure to air, an ethane–air mixture (reaction mixture) and pure ethane was recorded under conditions similar to those employed in the catalytic experiments. Adding increasing amounts of phosphorus to NiO changes its physicochemical characteristics; specifically, both the concentration and mobility of the surface lattice O− species in the NiO material decrease considerably, affecting its catalytic performance in ethane ODH. Thus, increasing the P content in NiO leads to a decrease in its catalytic activity with an increase in its ODH selectivity at the expense of total oxidation selectivity in the temperature range studied.
- This article is part of the themed collection: Nanocatalysis

 Please wait while we load your content...
Please wait while we load your content...