Removing the superfluous: a supported squaramide catalyst with a minimalistic linker applied to the enantioselective flow synthesis of pyranonaphthoquinones†
Abstract
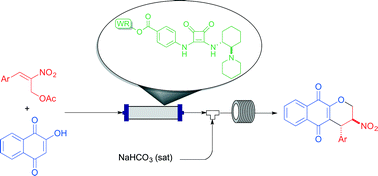
A continuous flow setup has been implemented for the enantioselective production of a library of pyranonaphthoquinones. This was possible through a sequential two-step process involving a squaramide-catalyzed Michael reaction and oxa-Michael cyclization. A key factor for the success of this methodology was the development of a new, cost-effective polystyrene-supported squaramide.
- This article is part of the themed collection: Catalysis in Flow Chemistry

 Please wait while we load your content...
Please wait while we load your content...