Supported rhenium nanoparticle catalysts for acceptorless dehydrogenation of alcohols: structure–activity relationship and mechanistic studies
Abstract
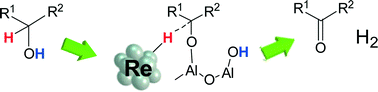
Al2O3-supported Re with different oxidation states and Re0 metal nanoparticles on various supports are prepared, characterized and tested for the dehydrogenation of 2-octanol. The activity of Re/Al2O3 increases with the fraction of metallic Re. The activity of metallic Re depends on the support oxides, and the support with moderate electronegativity (Al2O3) gives the highest turnover frequency (TOF) per surface Re0 site. Re/Al2O3 is effective for acceptorless dehydrogenation of various aliphatic secondary alcohols to ketones. The kinetic isotope effects on the dehydrogenation of 2-propanol show that dissociation of the α-C–H bond of 2-propanol is the rate-limiting step. The IR study of the reaction of gas phase 2-propanol over the Re/Al2O3 surface shows that the acid–base pair site of Al2O3 is responsible for the O–H dissociation of 2-propanol. The structural requirements are discussed on the basis of the mechanistic results.

 Please wait while we load your content...
Please wait while we load your content...