Direct dehydration of 1,3-butanediol into butadiene over aluminosilicate catalysts†
Abstract
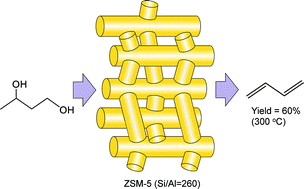
The catalytic dehydration of 1,3-butanediol into butadiene was investigated over various aluminosilicates with different SiO2/Al2O3 ratios and pore architectures. A correlation between the catalytic performance and the total number of acid sites and acid strength was established, with a better performance for lower acid site densities as inferred from combined NH3-TPD, pyridine adsorption and 27Al-NMR MAS spectroscopy. The presence of native Brønsted acid sites of medium strength was correlated to the formation of butadiene. A maximum butadiene yield of 60% was achieved at 300 °C over H-ZSM-5 with a SiO2/Al2O3 ratio of 260 with the simultaneous formation of propylene at a BD/propylene selectivity ratio of 2.5. This catalyst further exhibited a slight deactivation during a 102 h run with a decrease in the conversion from 100% to 80% due to coke deposition as evidenced by XPS and TGA-MS, resulting in a 36% loss of the specific surface area.
- This article is part of the themed collection: 2016 most accessed Catalysis Science and Technology articles

 Please wait while we load your content...
Please wait while we load your content...