Catalysts for direct H2O2 synthesis taking advantage of the high H2 activating ability of Pt: kinetic characteristics of Pt catalysts and new additives for improving H2O2 selectivity†
Abstract
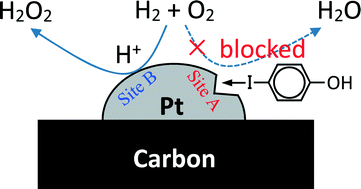
To develop efficient catalysts for the direct H2O2 synthesis from H2 and O2 by taking advantage of the high H2 activating ability of Pt, kinetic studies of the H2–O2 reaction were performed using a Pt-PVP (polyvinylpyrrolidone) colloid and Pt supported on carbon (Pt/C) as catalysts, and new additives were explored. Only 10−9 N of protons was sufficient to maintain the H2 reaction rate, suggesting that the dissociatively adsorbed H2 on Pt was very active. The addition of H+ and Br−, which is most effective for Pd catalysts, exerted much less influence on Pt, resulting in very low H2O2 selectivities. Inorganic and organic iodides as well as some sulfur compounds increased the H2O2 yield. The kinetic analysis of the reaction indicated that the H2O2 formation selectivity of Pt/C was maximized in combination with H+ and p-iodophenol, and was nearly comparable to that of Pd/C combined with H+ and NaBr; and indicated that the catalytic activity of Pt/C was higher. However, the much higher H2O2 destruction activity of Pt/C compared to that of the Pd catalyst spoiled the total catalytic performance of the Pt catalyst for H2O2 formation. Sulfur (Na2S2O3) and phosphine (tris(hydroxymethyl)phosphine) unselectively depressed the H2–O2 reaction.

 Please wait while we load your content...
Please wait while we load your content...