Mechanistic studies of the photo-electrochemical hydrogen evolution reaction on poly(2,2′-bithiophene)†
Abstract
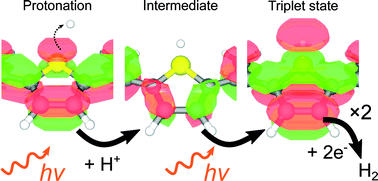
The realisation of poly(2,2′-bithiophene) (PBTh) as an effective photo-electrocatalyst for the hydrogen evolution reaction is a novel discovery [Ng et al., Int. J. Hydrogen Energy, 2014, 39, 18230]; however, the underlying mechanism of this catalysis remains unknown. In this article, studies using electrochemical, photo-electrochemical, Raman spectroscopy and computational modelling are undertaken to shed some light on the mechanistic process. From these studies, a compelling reaction scheme is proposed involving the protonation of the PBTh chain via the sulphur atom and subsequent intersystem crossing to a long-lived triplet state for the reaction to form H2. This suggested mechanism is tentative but cohesively integrates all experimental and computational findings. Importantly, these insights into the PBTh system form an important mechanistic milestone study and will help inspire new developments and applications for polythiophenes and conducting polymers.

 Please wait while we load your content...
Please wait while we load your content...